-40%
2019 Newest 12-channel ECG/EKG Holter System/Recorder Monitor Analyzer Software
$ 263.47
- Description
- Size Guide
Description
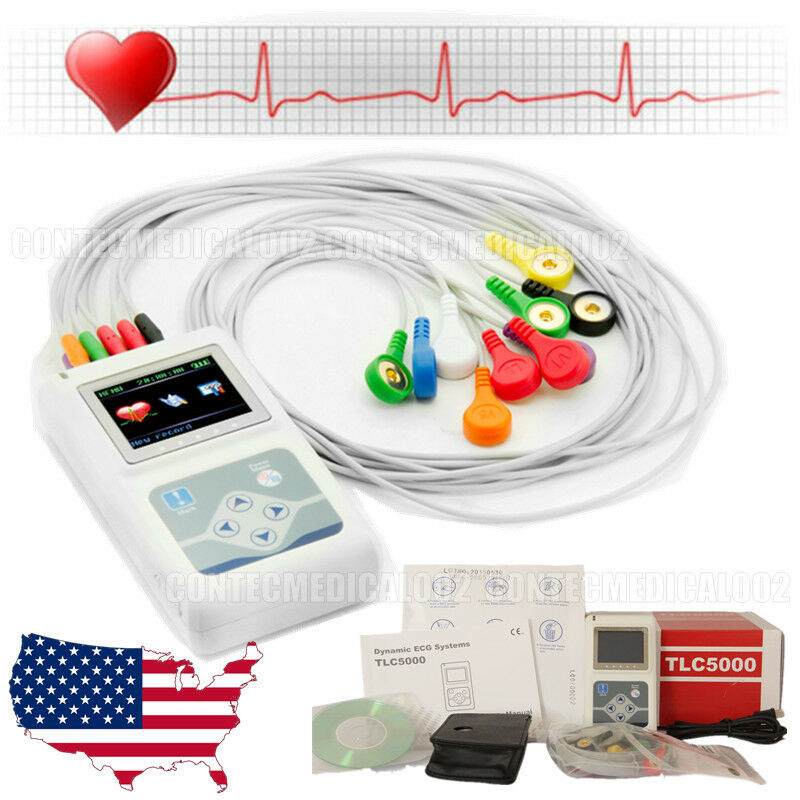
TLC5000 24Hours ECG Holter 12 ChannelsBrief Introduction
TLC5000 Dynamic ECG Systems adapts international standard 12-lead system, can record continuously ECG waves for 24h, and analyses waves with PC software. It is applicable in hospital and community medical establishment.
Recorder Features
With advanced design and technology, the recorder can work stably, dependably and durably.
Be provided with the capability of anti-jamming and aseismatic.
Small volume with OLED screen. The resolution is 160*128.
Be provided with functions of waveform preview, record review and event marking.
Accurate record of the start time of the data sampled owing to the real time clock of itself.
Avoid damage owing to the repeating inserting and pulling out by using the inbuilt TF card to storage the data.
The maximum capability can be 2GB, so the original data can be saved perfectly by no compression.
The average review time of single case can be less than 40 seconds by using interface of USB 2.0.
Record waveform details inextenso by the design of high precision and sampling frequency.
Record the state of pacemaker exactly by higher sampling frequency.
International standard of 12 leads. Record 24-hour DECG with the same standard of the ECG.
The features of software
Quick and accurate analysis system.
The 12-leads synchro analysis, to find accurate,QRS search can be accurate and no distortion.
There are more than 10 templates as atrial premature beat module, ventricular premature beat module, long interval module, atrial flutter module, atrial fibrillation module etc. and multiplicate User-defined templates could almost identify every kind of pathologic waveforms.
Flexible analysis channel selection function,can choose any channel as the mainly analysis the channel.
Flexible atrial fibrillation analysis ,so that physicians can use full,segmented automatic manual analysis of atrial fibrillation,make the analysis more quickly and accurately.
Powerful pacemaker analysis,on the AAI,VVI,DDD etc. all pacemaker analysis.
Many pacing modules have been added.
Fast review analysis function,can be any period of time the review single lead or all leads electrocardiogram.
Analysis of heard rate variability with short-range 5 minutes and long-range 1 hour and 24 hours analysis of heart rate variability.
Qne-stop printing operation,report printing process is convenient and quick.
Perfect function of case management.
Advanced ST segment analysis and myocardial ischemia total load,can for the ST abnormal analysis according to the whole and event,make the doctor can comprehensive judgment myocardial ischemia.
Unique "Sleep breath pause syndrome analysis" analysis can predict Sleep breath pause risk.
"HRT" analysis can predict the risk of death in patients with myocardial infarction.
"T Wave alternation" analysis is an important index to predict malignant arrhythmia and sudden cardiac death.
With QT discrete degree analysis,ventricular late potential analysis,vector ecg analysis,time vector analysis and so on,many kinds of additional analysis function,make analysis report more reference value.
Standard
Number of channels: 12 channels;
Sampling accuracy:12Bit
Record time : 24 h;
Power: 2 AA size batteries;
Interface: USB2.0;
Scale voltage:1mV±5%;
Standard Sensitivity:10mm/mV±5%;
Noise level:≤30μV;
CMRR:≥60dB;
Low-frequency characteristics : Time constant≥3.2s;
Scan speed:25mm/s±5%;
Enduring polarization voltage:±300mV DC polarization voltage,sensitivity shift≤±10%;
Least measure signal : 50 µV p-p;
Product safety type : Type B (Internally powered).
Accessories
12-lead ECG lead wires (1 set)
ECG electrode 1 bag(20 per bag)
USB data line(1)
Disk(1)
Bag(1)
User manual(1)
Softdog
Physical characteristic
Dimension:111mm(L) * 60mm(W) *25mm(H)
Weight:About 105g(without battery)
Certificate
CE FDA
Buy safe Products
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE:
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies.If the item is subject to FDA regulation, We will verify your status as an authorized purchaser of this item before shipping of the item.
If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA's Center for Devices and Radiological Health.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 197923,
and certified by FDA of United States and CE,TUV of Europe.The Fingertip Pulse Oximeter that is FDA 510K Approved
We only have the English Manual in the standard package, If you need any other language Manual, please contact us freely