-40%
Digital Color 6 Channel Electrocardiograph 12 Leads Touch EKG Cardiac Machine US
$ 210.67
- Description
- Size Guide
Description
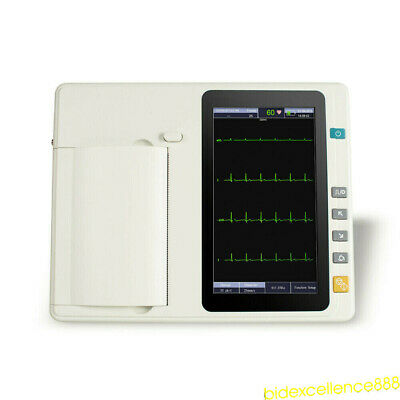
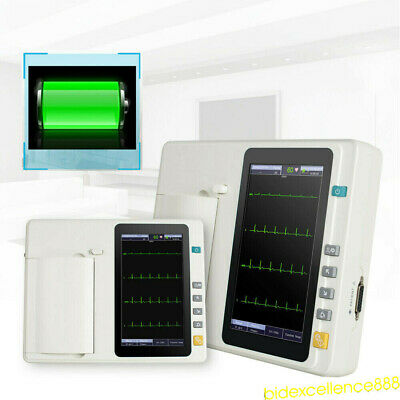
2Years Warranty!!!7 inch graphic LCD display,touch screen and function key control at the same time, more convenient and shortcut.
Six
channel ECG machine with interpretation
Auto-regulation of baseline drift can effectively inhibit baseline drift, optimizing the printing position to achieve high-quality ECG.
With automatically measuring and analyzing function for ECG parameters to reduces the doctor’s burden and improves work efficiency.
With a high resolution thermal printer to print out ECG trace,describing the trace clear and accurate,annotation as well as related parameters for diagnostic reference.
12-lead ECG simultaneous acquisition.
Adapt 110-240V, 50/60Hz AC power supply.
Specifications:
Lead
standard 12 leads
lead acquisition
synchronously
12 leads
Input circuit
Floating; Protection circuit against Defibrillator effect
Input Impedance
≥
50
M
Ω
Input circuit current
≤0.0.05μA
Record mode
Automatic: 3CH×4+1R, 3CH×4, 3CHx2+2CHx3,3CHx2+2CHx3+1R,6CHX2;
Manual: 3CH, 2CH, 3CH+1R, 2CH+1R;
Rhythm: Any lead selectable.
Filter
EMG Filter: 25 Hz / 30 Hz / 40Hz/75 Hz / 100 Hz / 150Hz
DFT Filter: 0.05 Hz/ 0.15 Hz
AC Filter: 50 Hz / 60Hz
CMRR
>100dB
Patient current leakage
<
10μA
Input Circuit Current
<0.05µA
Frequency Response
0.05Hz~150Hz (-3dB)
Sensitivity
2.5mm/mV, 5 mm/mV, 10 mm/mV, 20 mm/mV
Anti-baseline Drift
Automatic
Time constant
≥3.2s
Noise level
<15μV
p-p
Paper speed
12.5 mm/s, 25 mm/s, 50 mm/s
Recording mode
Thermal printing system
Paper specification
80mmx20m
paper
roll
LCD display
7”
graphic LCD
Safety classification
IEC60601-1 class I, type CF
Power supply
AC:
100~240V, 50/60Hz, 30VA~100VA
DC
: 14.8V/2200mAh, built-in lithium battery
Fuse
AC220V±10% 2*Φ5×20mm,T2A/250V AC time lag
AC110V±10% 2*Φ5×20mm,T4A/250V AC time lag
Package List:
ECG Machine×1
ECG Lead Wire×1
Limp Clip×4
Chest Suction Ball×6
Thermal Printing Paper×1
Fuse×1
Power Cable
×1
This product bears CE mark and the notified body is 0482
EC certificate No.: 6730GB410160520
Report No.: 6730FS06F
*Payment
1.We accept PayPal only. We will ship out the item after receiving cleared payment.
2.Please make sure you PayPal account is valid prior bidding.
3.Great appreciate for prompt payment. Unpaid item will be filed without receiving payment in 7 days after listing ended.
*Shipping
1.After full payment is received and cleared, we will ship it ASAP .
2.A complete and correct shipping address is essential.
3.Generally, mor than 200$,Expedited shipping !!!
*Return Policy
All items are with 2 Years warranty .
we accept returns, contact us within 60 days from delivery date and we will give you a full refund or exchange.
Only unused, undamaged and original condition item can be qualified for a return.
Buyer is responsible for return shipping fee.
*FDA declaration
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.(
The seller Name: Lily Lee, City: Beijing ,Country: ChinaTEL:15001145569)
This item has been cleaned and treated according to the manufacturer's instructions.
The Fingertip Pulse-Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 136606, and certified by FDA of United States (Premarket Submission Number (510K): K073454 Listing Number: D045684, K082641 Listing Number: D064765, K090671 Listing Number: D078664); and CE Approved, TUV of Europe (Cert.No. : G1 10 02 50972 013). You can consult with the FDA's Center for Devices and Radiological Health.
*Contact Us
Any questions, please contact us through eBay message, We will reply within 1 business days (public holidays not included).
Our working time: Monday - Friday 9:00 AM - 5:30PM (Beijing)