-40%
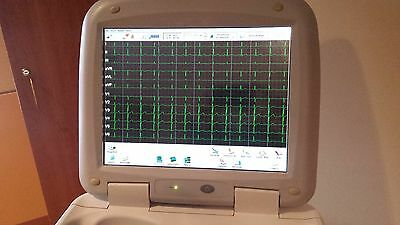
Philips PageWriter Touch 12-Channel EKG
$ 633.6
- Description
- Size Guide
Description
The Philips PageWriter Touch 12-Channel EKG with Kart is in excellent condition, and certified to proper operating specifications.Precision performance and sophisticated ergonomics converge on the Philips PageWriter Touch EKG, making it easier to record, analyze, print, store, and transmit ECGs. Designed for use in large hospitals where high quality and accuracy are paramount, PageWriter Touch makes every step in the testing process easy.
Philips PageWriter Touch 12-Channel EKG Features:
EKG Acquisition: EKG signal acquisition of up to 12 leads for both adult and pediatric patients.R/T (real-time) 12-lead display with EKG "snapshot" feature: Take EKG snapshots of any data on the screen with the touch of a button - review snapshots and print EKG reports in a variety of formats.Auto 12-lead acquisition. Rhythm display and printing (up to 12 leads). Disclose 5 minutes of continuous waveform data for review and printing.
EKG Quality Monitor:
Extensive ECG quality indicators include AC noise filtering, in addition to artifact filtering and baseline wander correction. Color-coded real-time waveform display and loose electrode alerts provide instant quality feedback to the user.
EKG Memory and Transfer:
The PageWriterTouch cardiograph provides storage memory for ECGs, and easy transfer by floppy disk, modem, or LAN connection to an ECG management system. Each recorded ECG is automatically indexed on the cardiograph for quick review and retrieval. A list of all stored ECGs can be printed, facilitating administrative reporting and billing. The optional modem allows for quick transmission to a fax machine or an automated ECG management system. ECGs may be transmitted for management, physician review, and copying.
EKG Interpretation:
The PageWriterTouch cardiograph includes the Philips 12-Lead Algorithm for interpretive analysis of the amplitudes, durations, and morphologies of ECG waveforms and associated rhythm for adult and pediatric patients.
This clinically proven interpretation program provides configurable levels of interpretive, reason, and severity statements that are printed on the ECG report.
Print Preview Capability:
Full-screen preview of EKG reports exactly as they appear when printed allows for quality assessment checks prior to printing.
Multiple report formats may be applied to any EKG, with full preview of reports before printing.
Battery:
Rechargeable battery records approximately 50 EKGs or 40 minutes of rhythm recording from a single battery charge.
Philips PageWriter Touch 12-Channel EKG Specifications:
EKG Acquisition: R/T (real-time) ECG (12 leads), Auto (12 leads), Rhythm (up to 12 leads), Disclose (1 to 3 leads).
Keyboard: Full alphanumeric capability.
Touch Screen Display: 1024 x 768 pixel resolution. 30.4 cm x 22.8 cm (15" diagonal) color liquid crystal touch screen display with backlight.
Patient Module: Action button allows user to take EKG snapshots from the bedside.
Signal Processing/Acquisition Sampling Rate: 1,000 samples per second per electrode/lead. 12 bit A/D conversion provides 5 µV resolution.
Auto Frequency Response: 0.05-150 Hz, 0.15-150 Hz, 0.5-150 Hz, 0.05-100 Hz, 0.15-100 Hz, 0.5-100 Hz, 0.05-40 Hz, 0.15-40, 0.5-40 Hz.
Rhythm Frequency Response: 0.05-150 Hz, 0.15-150 Hz, 0.05-100 Hz, 0.15-100 Hz, 0.05-40 Hz, 0.15-40 Hz.
Filters: AC noise, Baseline wander, Artifact.
Printer/Printer Resolution: High-resolution, digital-array printer using thermal-sensitive paper, 200 dpi (voltage axis) by 500 dpi (time axis).
Report Formats: 3 x 4 (1R, 3R), 6 x 2, Panoramic 12 (Cabrera), 12 x 1, Rhythm (up to 12 selected leads).
Extended Measurements.
1 Minute Disclose (1 lead).
Full Disclosure (5 minutes, 1 to 3 selected leads).
Battery Operation Capacity: Typically 50 ECGs and copies on a single charge or 40 minutes of continuous rhythm recording recharge. 7 hours in standby mode to >90% capacity (typical).
Network Connection: 10/100 Base-T IEEE 802.3 Ethernet via RJ45 connector (standard).
Optional software required for wireless LAN connection (Wireless PC LAN card not included).
ECG Storage: 150 ECGs to internal flash memory. 2 to 3 ECGs typical per 1.4 MB floppy disk.
ECG File Formats: XML and XML SVG.
Power Requirements: 100-240 Vac, 50/60 Hz, 150 VA max.
Operating Conditions: 50-104° F (15-35° C) /15-70% relative humidity (non-condensing) /Up to 15,000 ft (4,550 m) altitude.
Storage Conditions: 0-122° F (0-40° C), 15-80% relative humidity (non-condensing). Up to 15,000 ft (4,550 m) altitude.
Dimensions and Weight: Approximately 17.7" x 18.0" x 6.34" (45 cm x 45.8 cm x 16 cm).
Approximately 28 lbs (13 kg) including accessories.
Patient Interface Module: Remote, microprocessor-controlled module.
RETURN POLICY:
All equipment shipped is tested to proper operating and cosmetic specifications. All equipment shipped is protected by paid for carrier insurance. Providing equipment shipped was nonconforming to invoice, equipment can be returned for refund.
INTERNATIONAL SHIPPING:
If you wish to have your medical equipment shipped outside of the US, please contact us prior to purchasing your item with your international address in order to receive exact shipping costs. International shipping is not free, and the shipping costs will be paid for by the buyer. International shipping costs will vary by the location where the item is being shipping.
Disclaimer on all medical category auctions.
"The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item."
All equipment has been handled and cleaned per the manufacturers instructions. All equipments is tested to manufacturers specifications.
Buyer accepts full responsibility and will not hold seller or Ebay responsible for inappropriate use of this device. Once purchased the buyer accepts full responsibility and all liability from the use of this device