-40%
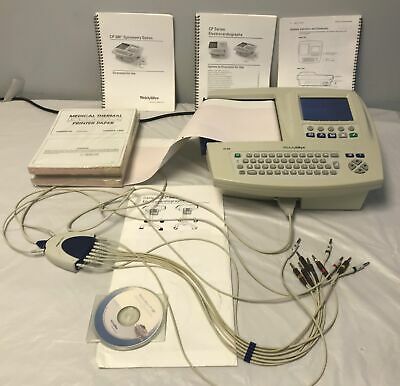
Welch Allyn CP 200 EKG Spirometry Interpretive ECG Machine
$ 475.2
- Description
- Size Guide
Description
Welch Allyn CP 200 EKG Spirometry Interpretive ECG MachineUpon receipt, as with all clinical devices, it is the BUYER'S responsibility to have this instrument checked and re-certified for medical use by a qualified Biomedical Technician prior to placing into service.
Cosmetic condition is Good
This item is USED and in good cosmetic condition. It displays signs of wear, such as minor to moderate scratches or scuffs.
Functionality
Item has been tested for Power up only. No further testing was done.
Includes
Item as pictured
1 Welch Allyn CP 200 EKG Spirometry Interpretive ECG Machine
Lead Cords
Extra Paper
Manual with CD
Item as pictured- Nothing Else Included
Not Included
Anything else
Video
Click Here or copy / paste the link to see Video of our items working
https://www.youtube.com/watch?v=lD8uboQNP0w&feature=youtu.be
Additional Information
This FDA DISCLAIMER is for MEDICAL DEVICES only and not for all our products: The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, we will verify your status as an authorized purchaser of this item before shipping of the item.
NOTE: BY PURCHASING THIS SYSTEM, THE BUYER AGREES TO RELEASE Zekic LLC FROM ANY AND ALL LIABILITY PERTAINING TO THE FUTURE USE OF THIS SYSTEM.
Upon receipt, as with all clinical devices, it is the BUYER'S responsibility to have this instrument checked and re-certified for medical use by a qualified Biomedical Technician prior to placing into service.